Home > Our Capabilities | Technology Portfolio > 2. Ni catalyzed Suzuki-Miyaura Coupling
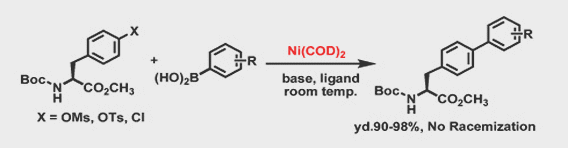
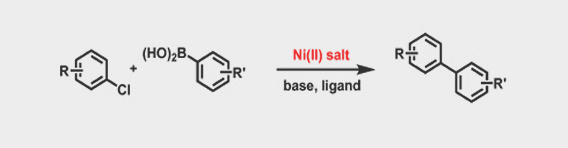
- The use of highly reactive catalyst such as Ni(COD)2 as well as cheap and more convenient divalent nickel catalyst
- Excellent yields were observed with reactions at room temperature, which eliminates racemization in a wide variety of substrates
- The use of inexpensive leaving groups such as OTs, OMs or Chlorides
- Negligible Ni residue in the product without special treatment
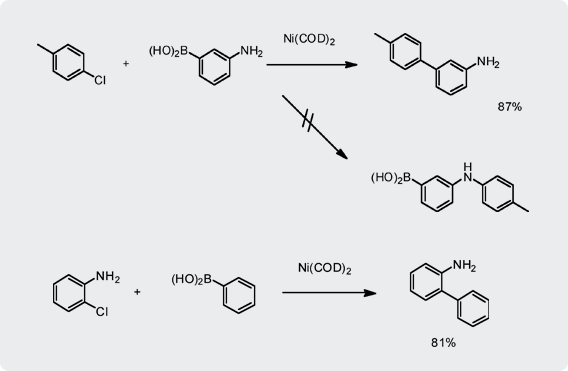
- Ni catalyst tolerates free amino group on either boronic acid or halide for Suzuki coupling.
- More extensive research work by Sumitomo Chemical shows no competition with Buchwald type amination reaction of halides.
- Again there is no concern about residual Ni in the products.
- Quick catalyst screening to identify the best catalyst system for each substrates at our laboratory
- Metal species (Ni, Pd)
- Ligands
- Base
- Established procedures for preparation / handling of air sensitive catalysts such as
Ni(COD)2, Pd(PPh3)4 - In-house manufacture of boronic acids which enables to simplify total supply chain of Suzuki coupling products

