Home > EHS & Quiality | Quality Assurance
Each plant is equipped with advanced facilities for cGMP operations. Our commercial scale API production sites are equipped with purified water system, microbial testing facilities, GMP compliant warehouses.
Our quality culture is exemplified in excellent inspection history by U.S. FDA and Japanese authority . The capabilities of Sumitomo Chemical to respond to cGMP requirements are well recognized internationally.
| Gifu | Okayama | Utajima | Oita |
|---|---|---|---|
| 2016 2014 2010 2009 2005 2003 2000* 1997 1996 1991 1987 1984 1982 1980 1978 1975 |
2016 2011* 2004* |
2016 2009* 2005* 1997 1994(×2) 1993 |
2016 2012 2004* 1997 1994 1990 1988 |
| *) Note: Zero 483 form was issued in 2011 (Okayama), 2009 (Utajima), 2005 (Utajima), 2004 (Okayama & Oita) and 2000 (Gifu) | |||
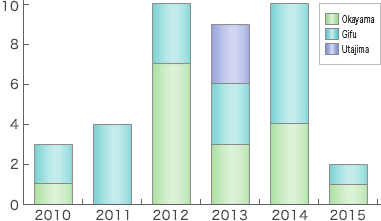
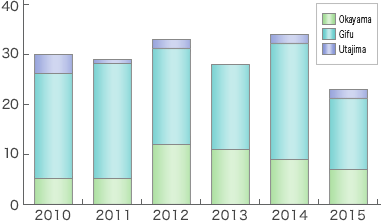
Sumitomo has abundant experience in filling and maintaining DMF in Europe and the U.S.A. We have accumulated know-how for preparing CMC (Chemistry Manufacturing Control) parts. With such knowledge and experience, we serve customers for their filing new drug applications for approval.
- DMF submission
6 products (USA), 1 product (CAN), 4 products (EU), 3 products (OCE), 2 products (GCC), 1 product (KOR), 1 product (TWN), 42 products (JPN) *) including 4 discontinued products - Making necessary reports
- Identification of critical process parameters
- Providing information on drug substance / intermediates
chemical structure, general physicochemical properties, particle size, polymorphism

